https://www.englisch-hilfen.de/en/exercises/questions/mistakes.htm
More than 20 million tons of salt are used every year to melt snow and ice in cold northern regions. But how does salt do it?
it’s a cheap and effective way to protect roads from ice due to a simple scientific principle: freezing point depression of solutions. The freezing point of pure water, the temperature at which it becomes ice, is 32 degrees Fahrenheit. So if there’s snow, sleet or freezing rain and the ground is 32 F or colder, solid ice will form on streets and sidewalks.
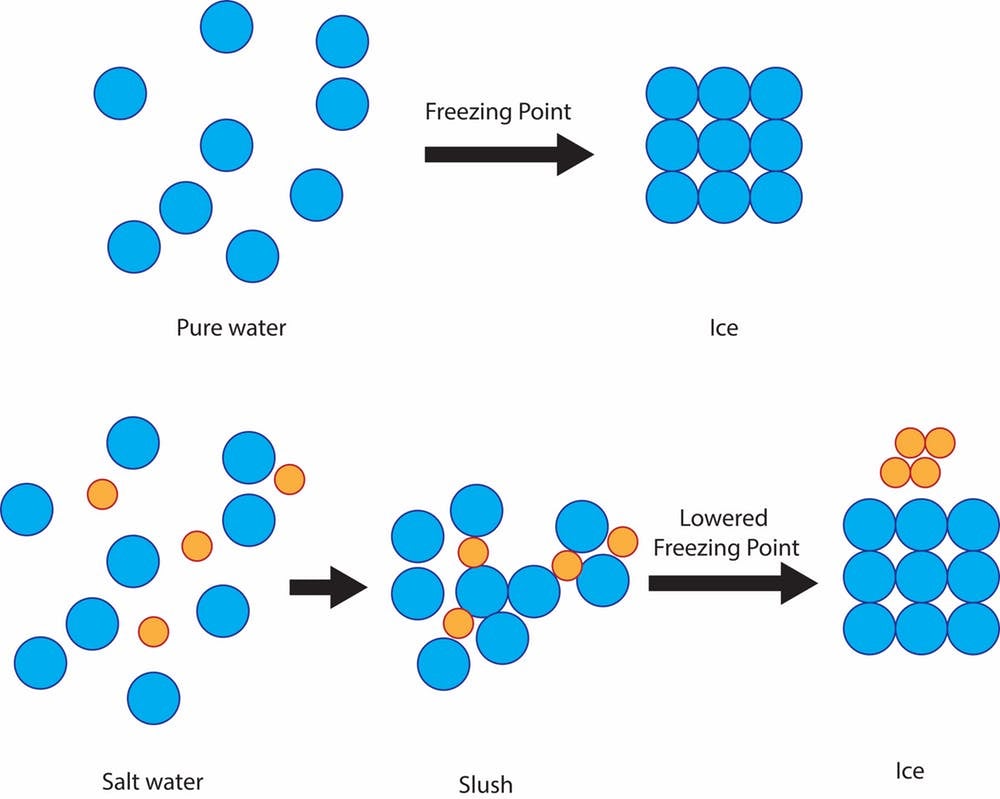
If the water is mixed with salt, though, the freezing temperature of the solution is lower than 32 F. The salt impedes the ability of the water molecules to form solid ice crystals. The degree of freezing point depression depends on how salty the solution is.
It’s important to note that the salt must be in a solution with liquid water in order for this principle to be obeyed. That’s why many cities spray a salt solution before any ice forms
Комментариев нет:
Отправить комментарий